Last Updated on October 24, 2023 by Kevin Chen
Heat and temperature are two terms that are closely related hence confusing. In this guide, we are going to analyze the differences and similarities of heat vs temperature.
Definition:heat vs temperature
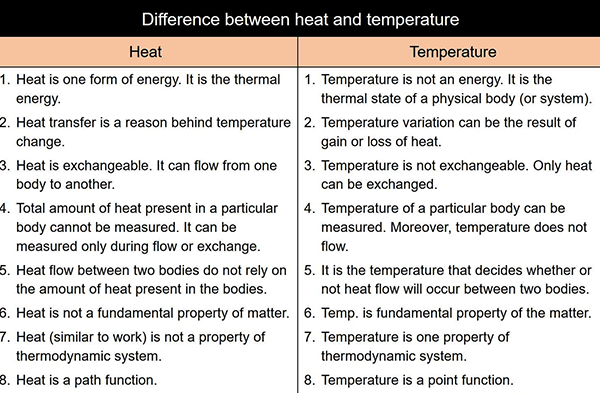
Heat is a form of energy that is transferred from one body to another body, this process is known as conduction. The capacity to transfer heat from one body to another is dependent on the material in question, for example, metals are good conductors of heat while plastics are not.
Temperature is the measurement of heat in a body. The temperature of a body is usually measured by using a thermometer that contains mercury or alcohol.
Units of measurement: Heat vs temperature
Heat
The SI unit of measurement of heat is Joule (J) and the CGS unit is Calorie (cal).
Temperature
The SI unit of measurement of temperature is Kelvin (K) and the CGS unit is Celsius degree (°C).
Ability to work
Heat has the ability to work and convert energy. On the other hand, temperature cannot work.
The device of measurement: heat vs temperature
From the properties, we can tell that temperature and heat are measured using different devices.
Temperature is measured using a thermometer while heat capacity is measured using a calorimeter.
Calorimeter
A calorimeter is used to measure the heat capacity of gas, the device consists of two chambers.
The first chamber is called the cold chamber which contains the gas and its surroundings. The second chamber is called a hot chamber which contains a known amount of gas and its surroundings.
The temperature of both chambers is controlled by a thermostat to maintain them at a constant temperature.
The difference between temperatures in both chambers is measured by a thermometer and the measurement is repeated until a steady-state condition is achieved.
Thermometer
The thermometer is used to measure the temperature of the gas, liquid, solid, and surroundings.
The thermometer consists of two parts.
The first part is called the bulb and the second part is called the thermometer tube.
The bulb contains the gas and its surroundings while the thermometer tube contains the mercury which is used to measure temperature.
The bulb has a small hole in it through which mercury passes. The mercury then expands due to the temperature rise, it flows down till the end of the thermometer where it touches the glass bulb which acts as a mirror for the reflection of light from mercury. By this method, we can calculate the temperature at different places by measuring its distance from mercury.
What does the hotness of the body mean?
The hotness is usually used in reference to thermodynamics. The hotness of a body means the amount of heat it can release or absorb.
The hotter the object is, the more heat it can be able to release or absorb.
The colder the object is, the less heat it can be able to release or absorb.
The term hotness is closely related to the empirical temperature. This is the temperature at which something is considered hot.
The empirical temperature is the temperature that is measured by the human senses.
The empirical temperature can be measured by using a thermometer or any other similar device.
The empirical temperature does not represent the true heat of a body but it is a good approximation of it.
The hotness of a body depends on its ability to release or absorb heat and its energy content.
Is there any difference between hotness and heat?
The hotness of an object is a measure of its ability to release or absorb heat.
Heat is a form of energy that can be used to do work or be converted into another form of energy.
The energy content (the amount of energy) depends on the mass and the heat capacity (the amount of heat that can be absorbed by a body) depends on the temperature.
Relationship between empirical temperature and heat
The empirical temperature is a measure of the ability of a body to release or absorb heat.
The empirical temperature can be measured by using a thermometer or any other similar device.
The empirical temperature does not represent the true heat of a body but it is a good approximation of it.
What are theoretical scales of temperature?
Theoretical scales of temperature are scales that have been developed to measure the true heat of a body.
Theoretical temperatures can be measured by using a thermometer or any other similar device.
Theoretical temperatures are not directly measurable but they are related to empirical temperatures by using empirical data and statistical methods.
Statistical methods can be used to make theoretical temperatures more accurate.
An example explaining the difference between heat vs temperature
If the room temperature is 20°C and you place a piece of ice in the room then that will make the temperature rise to 0°C.
This is because heat is being transferred from the air to the ice.
The air around the piece of ice will exchange heat with it so that the temperature of both air and ice will be 0°C.
The only thing that has changed is that now there is no heat being transferred from air to ice but instead heat is being transferred from the air to a warm body (in this case human).
Heat transfer between two objects with different temperatures can be described as follows
Q – Heat transfer between two bodies with different temperatures, T – Temperature difference between two bodies, W – Heat capacity of a body.
Q = W/(T-T)
where:
Q = Heat transfer, W = heat capacity of one body, T= temperature of one body, T-T = temperature difference between two bodies.
If the temperatures are equal then there is no heat transfer.
Let us now consider the same situation but this time instead of a piece of ice we will have a black box and instead of the air we will have gas.
Conclusion
In conclusion, heat and temperature are two important factors that are needed to understand the world around us. Heat is a form of energy that is transferred from one object to another.
These two objects can be two different materials such as metal and glass, or two different gases such as water and air. In all these cases, heat is required to take place for an object to change its temperature.
If you want to find more Electronic Components Distributors, please check out the following articles:
Electronic Components Distributors In the USA
Electronic Components Distributors In UK
Electronic Components Distributors In China
Electronic Components Distributors In India
Electronic Components Distributors In Singapore
Electronic Components Distributors In Malaysia
Electronic Components Distributors In Vietnam
Electronic Components Distributors In South Korea
- Where to buy IC chips? The Best Guide? - March 26, 2024
- Breaking Down Barriers: Overcoming Obstacles in Cross-Border Electronic Component Trade - March 4, 2024
- Everything You Need to Know About Amplifier IC Chips - March 4, 2024