Last Updated on October 22, 2023 by Kevin Chen
A supercapacitor is a power storage device that combines the qualities of capacitors and batteries into one device, resulting in a very large capacitance. These capacitors have a higher energy storage capacity than other capacitors and higher output power than batteries.
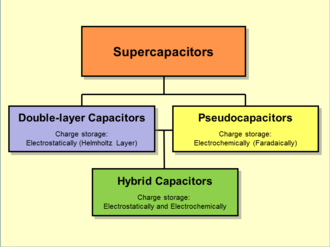
Supercapacitors are a type of capacitor that is easy and safe to use. Pseudocapacitors, Electrostatic double-layer capacitors, and Hybrid capacitors are the three types of supercapacitors accessible, depending on the application. The working and applications of one of the varieties of supercapacitors, the Pseudocapacitor, are discussed in this article. But first, let us refresh our memories on what capacitors are and what they perform.
What is a Capacitor?
As a result, a capacitor, also known as a condenser, is a combination of two conducting plates separated by a dielectric or insulating layer. The main purpose of this electrical component is to store electrical energy. The size and geometrical arrangement of the plates and the type of dielectric material utilized varied between capacitors. Ceramic, Mica, air, paper, and electrolytic capacitors are some of their names.
For use in tuning circuits, their capacitance can be adjustable or set over a range of values. For instance, the effort done (by a battery) in establishing opposite charges on the two plates at the supply voltage corresponds to the energy stored by a capacitor.
The size of the plates, the dielectric material in the space, spacing, and the voltage applied to define the amount of charge that may be stored. Each half-cycle, a capacitor in an AC circuit is alternately charged and discharged. The amount of time available for charging or discharging is thus determined by the frequency of the current, and if the time required exceeds the half-cycle length, the polarization (charge separation) is incomplete.
In such a circuit, the dielectric constant seems smaller than in a direct-current circuit and changes with frequency, decreasing at higher frequencies. The charges must be alienated through the dielectric first in one direction and then in the opposite direction during the alternation of polarity of the plates, and overcoming the opposition results in the generation of heat known as dielectric loss. When using capacitors in electrical circuits such as radio and television receivers, this feature must be regarded: the frequency and dielectric material influence dielectric losses.
When a capacitor is subjected to a constant voltage, no current flows through it except for a small amount of leakage through the dielectric. On the other hand, a displacement current is an alternating current that passes easily.
What Is a Capacitor Used For?
In today’s world, capacitors are everywhere. These components aren’t visible, but they’re present in almost every electrical and electronic gadget you use. So, what is the function of a capacitor in these devices?
Let’s look at a few of the most frequent capacitor uses.
Camera flashes
Camera flashes used small filament bulbs to generate light until LEDs became popular. Without an excessively huge body, a historical camera couldn’t produce enough power to produce a powerful flash. Capacitors solved this issue by charging and storing energy before each flash. Pulsed power is demonstrated in this way.
Computers
When a volatile storage device, such as RAM loses power, the data it contains is lost. When power supplies need to be replaced, this causes difficulty, but a capacitor can address the situation by providing temporary power. An example of power storage is this.
Analog Stereo Equipment
Creating clean amplifiers, audio, and other analog stereo equipment requires precision electronics. In circuits like these, it eliminates hum, capacitors, smooth current variations, and undesired noise. This is a case of power conditioning in action.
What is Pseudocapacitor
Pseudocapacitors use electron charge transfer between electrode and electrolyte to store electrical energy radically. Pseudocapacitance is achieved through reduction-oxidation, electrosorption, reactions, and intercalation processes.
When an electrochemical capacitor is combined with an electric double-layer capacitor, a pseudocapacitor is created.
A supercapacitor’s indivisible capacitance value is the sum of double-layer capacitance and pseudocapacitance. However, depending on the electrode design, they can be effective with varying portions of the total capacitance. Compared to a double-layer capacitance on the same electrode surface, a pseudocapacitance can be 100 times larger.
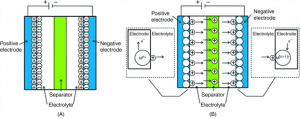
The electrical charge is stored electrostatically in an EDLC without interaction between the electrode and the ions. Moreover, a pseudocapacitor has a chemical reaction at the electrode. A desolvated and adsorbed ion causes pseudocapacitance. Furthermore, an electron charge is transferred between the electrolyte and the electrode. There is one electron in each charging unit. The adsorbed ion has no chemical interaction with the electrode’s atoms since only a charge transfer happens. During charging, consider a redox reaction under which the ion is Oxygen gas and one electrode hosts a reduction process whereas the other hosts an oxidation reaction. During discharge, the responses are reversed.
Unlike batteries, electron charge-transfer ions cling to the atomic structure of an electrode in faradaic electron charge transfer. This faradaic energy storage system relies solely on quick redox reactions and allows charging and discharging much faster than batteries.
Electrochemical pseudocapacitors use conductive polymer electrodes or metal oxide with a high electrochemical pseudocapacitance. In a linear relationship, the amount of electric charge stored in a pseudocapacitance is proportional to the applied voltage. Pseudocapacitance is measured in farads.
Pseudocapacitor Diagram
A pseudocapacitor combines a battery and an electric double-layer capacitor. This capacitor consists of two electrodes separated by an electrolyte.
Chemical and electrostatic processes are the most common methods for storing charge.
The chemical process mostly entails charge transfer via Redox or reduction-oxidation processes. When the charge transfer is similar to that of a battery, transfer rates are superior because the redox material over the electrode is thinner, allowing more ions from the electrolyte to diffuse into the structure. Because numerous processes operate to store charge, the capacitance values of pseudocapacitors are higher.
Working Principle
Pseudocapacitor transports electron charge between electrode and electrolyte. This is done via reduction-oxidation reactions, electrosorption, and intercalation processes. When a pseudocapacitor is combined with an electric double-layer capacitor, a supercapacitor is formed. When an electrochemical capacitor is connected with an electric double-layer capacitor, the result is a supercapacitor.
Metal hydroxides, metal sulfides, metal nitrides, metal oxides, and conducting polymers are commonly used in pseudocapacitive materials. Metal oxides such as NiO, RuO2, Co3O430, and MnO2 are pseudocapacitor materials. Pseudocapacitor materials include conducting polymers like polypyrrole and polyaniline. Electrical energy is stored in Pseudocapacitors during faradaic processes. As a result, they electrostatically store charge, allowing charge transfer between electrolyte and electrode. When a voltage is applied to a pseudocapacitor, the electrode material experiences combined reduction and oxidation. The faradic approach used in these capacitors, as opposed to EDLCs, will enhance electrochemical processes, leading to higher particular capacitance and energy densities.
The energy density of the materials used in pseudocapacitors rises, allowing for increased power storage density in the number of electrode materials on their surface. The essential factors of these capacitor materials are that they are electrically conductive and can oxidize in two different ways within a given potential window.
Due to their unique storage charge principles, these capacitors have the maximum capacitance density compared to other capacitors. As a result, the amount of electric charge stored in a pseudocapacitance is proportional to the applied voltage.
Faradaic Reaction
Because this reaction is primarily guided by Faraday’s law, which clearly says that the sum of a chemical reaction generated by current flow is proportionate to the quantity of electricity passed, electron transfer might result in oxidation or reduction.
Redox Reaction
When electrons are transferred between two reactants, it is called a redox reaction. By observing changes in the oxidation conditions of the reacting species, these electron transfers can be determined.
How to Connect a Pseudocapacitor?
The Pseudocapacitor’s circuit is depicted below. As illustrated in the diagram, ‘Rs’ is the series resistance. The voltage mostly determines the pseudocapacitance ‘C,’ ‘RD’ is the Faradaic resistance, which can be used throughout the discharge until the ions have vanished, and ‘RF’ is the electrode-electrolyte resistance. As a result, the pseudocapacitance surpasses the dual-layer capacitance ‘Cdl’ at specific potentials. According to the additive law of capacitance, the parallel arrangement of capacitors will considerably assist in increasing the capacitance of the Pseudocapacitor.
Types of Pseudocapacitor
Pseudocapacitors are divided into two varieties based on the electrode materials employed to store their charge, as shown below.
- Metal oxide
- Conducting Polymers
Metal Oxide
The metal oxide is a pseudocapacitive substance that exhibits reversible and quick redox reactions on the electrode materials’ surfaces. Because this material has a low resistance and a high specific capacitance, making supercapacitors with high power is simple.
MnO2, RuO2, IrO2, NiO, Fe3O4, SnO2, V2O5, Co2O3, and MoO are the most often utilized metal oxides as supercapacitor electrodes. Due to its excellent electrical conductivity and specific capacitance, RuO2 is considered the most capable metal oxide electrode material in supercapacitor applications. However, the lack of ‘Ru’ on the planet has limited its application. It is vital to recognize low-cost pseudocapacitance materials to address this major issue.
At different potentials, metal oxides display varying oxidation states and have crystalline structures that maximize conductivity, allowing charges to communicate in their network. During oxidation and reduction surface processes, protons could add to and remove from the oxide lattice, allowing metal oxides to change their oxidation conditions.
Conducting Polymers
Conducting polymers are employed in redox pseudocapacitors due to their quick and reversible oxidation or low-cost reduction processes and high electrical conductivity.
Polythiophene, Polypyrrole, Polyaniline, and p-Poly p-phenylene vinylene are the most often used conducting polymers and Polyphenylene Vinylene. These materials are typically made by chemical oxidation of the monomer or electrochemical oxidation, then made conductive by a conjugated link system and the polymer backbone.
Compared to carbon-based electrode materials, conducting polymer polymers offer increased conductivity, greater capacitance, and lower equal series resistance. Ions are detected or travel from the electrolyte to conducting polymers, where they are oxidized and reduced before returning to the electrolyte.
Due to the lack of a phase transition, these polymers have exceptionally reversible effects, improving cycling stability. They are charged positively or negatively through redox processes, resulting in increased conductivity.
Advantages and Disadvantages of Pseudocapacitors
Advantages
The following are some of the benefits of using a pseudocapacitor.
● These capacitors have a higher power density.
● They live for far longer periods.
● They charge and discharge much faster than lithium-ion batteries.
● The pseudocapacitor materials will increase energy density and allow power density storage within the bulk and at the surface of electrode materials.
Disadvantages
The following are some of the Pseudocapacitor’s disadvantages.
● Because these capacitors have a lower energy density, they cannot be used in energy storage applications in place of batteries.
● They aren’t appropriate for long-term energy storage.
● The output voltage of these capacitors does not increase linearly with their charge.
Applications Of Pseudocapacitors
The following are some of the uses for a pseudocapacitor.
- Pseudocapacitors use a faradaic reaction to store electrical energy.
- It’s a component of an electrochemical capacitor that combines an electric double-layer capacitor with an electrochemical capacitor to create a supercapacitor.
- Consumer electronics make use of these.
- Electronics that can be worn or flexed
- In-car applications, regenerative braking is used.
- wind turbines, Cranes, elevators, and other kinetic energy recovery systems
Last But Not Least.
Pseudocapacitors arose from the discovery that porous, high-surface-area materials like Ruthenium oxides, Manganese oxide, and other oxides had higher capacitance than EDLCs. The characteristic was dubbed “pseudocapacitance” after it was observed that some aqueous electrolytes and these compounds have reversible electrochemical processes.
Although much research has been done and continues to be done on pseudocapacitors, electrochemical reactions reduce the cell’s power and lifetime, and this problem has yet to be solved. As a result, EDLCs only store physical energy. Moreover, pseudocapacitors store both chemical and physical energy, making them supercapacitors.
Conclusion
This is a quick overview of Pseudocapacitor and its interaction with other programs. EDLC materials can’t increase energy density enough, but pseudocapacitor materials can. They increase energy density and allow energy density storage both inside and outside electrode materials. Pseudocapacitor’s key advantages include lightness, environmental friendliness, cost-effectiveness, comfort, flexibility, increased safety, and customizable electrochemical characteristics.
For more details or purchase of pseudocapacitor, contact us at ICRFQ. We manufacture the best electrical components in China.
If you want to find more Electronic Components Distributors, please check out the following articles:
Electronic Components Distributors In the USA
Electronic Components Distributors In UK
Electronic Components Distributors In China
Electronic Components Distributors In India
Electronic Components Distributors In Singapore
Electronic Components Distributors In Malaysia
Electronic Components Distributors In Vietnam
Electronic Components Distributors In South Korea
- Where to buy IC chips? The Best Guide? - March 26, 2024
- Breaking Down Barriers: Overcoming Obstacles in Cross-Border Electronic Component Trade - March 4, 2024
- Everything You Need to Know About Amplifier IC Chips - March 4, 2024